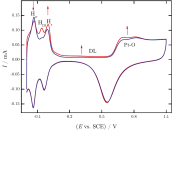
Generation and electrochemical nanogravimetric response of the third anodic hydrogen peak on a platinum electrode in sulfuric acid media
The paper authored by
B.B. Berkes and
G. Inzelt
is published in Journal of Solid State Electrochemistry (2014, vol. 122, pp. 11–15).
Abstract:
Platinum electrodes have been investigated in sulfuric acid solutions in the hydrogen adsorption–desorption region by electrochemical quartz crystal nanobalance (EQCN). It was found that a well-developed peak (the socalled third peak) between the two main peaks appeared when, following the cycling in the oxide region, the electrode was kept at potentials just more positive than the potential of hydrogen evolution under the same conditions. The extent of this third peak and its ratio to oxidation peaks of the strongly and weakly adsorbed hydrogen depend on the waiting time at potentials mentioned above as well as on the scan rate. Similarly to the other two peaks, the simultaneous EQCN response shows a slight mass increase which can be assigned to adsorption of HSO4– ions at the platinum surface. Because the third peak appears only after a potential excursion in the oxide region, it is related to the formation of specific surface sites on which hydrogen can be adsorbed with an energy which falls between the energies of the weakly and strongly bound hydrogen. The waiting time effect indicates that this adsorption is a slow process, and it is the very reason that it cannot be observed during the second cycle. The scan rate dependence can be elucidated by the transformation of this type of adsorbed hydrogen to the other two forms.
